

Review Article
Ricardo Madeira Research Center In Sport Sciences, Health Sciences And Human Development (CIDESD), Covilhã, Portugal.
Henrique P. Neiva
Adriana Maia
Dulce Esteves
Recieved on: 2023-10-20, Accepted on: 2023-12-18, Published on: 2023-12-27
Concurrent training is increasingly being recommended for healthy individuals, as well as for people with some special health conditions such as cancer. This update of the scientific evidence was carried out to synthesize and analyze data on the physical and psychological effects of concurrent training in cancer survivors. We searched three databases (PubMed, Web of Science, and Scopus) until June 2023 for systematic reviews and meta-analyses in cancer survivors. The initial search identified 2181 studies in 3 different databases. After removing duplicates, 1660 articles were identified. 201 articles were selected for full reading. Nevertheless, only one study satisfied the inclusion criteria on the effect of concurrent training on cancer pathology. The main conclusions regarding the effects of concurrent training on cancer were that it had positive effects on increasing peak oxygen uptake, maximal oxygen consumption, and decreasing triglycerides. However, there were no significant differences in heart rates (peak heart rate, peak respiratory exchange ratio, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol, and body mass index with the use of concurrent training. The intervention of concurrent training is scarce, so there is a need for original studies investigating the main characteristics and positive effects of concurrent training.
Cancer is the second main cause of death in the world [1]. According to the International Agency for Research on Cancer (IARC), one in five people develops this disease during their lives, which is why this is one of the public health issues that generates great concern. In 2020, there were around 19.3 million cases reported worldwide and by 2040 an increase to 28.3 million cases worldwide is expected [2].
Scientific and technological advances in the treatment of cancer have seen survival rates rise by leaps and bounds and, with this increase, issues related to the long-term care of survivors in psychological, physical, and quality-of-life terms are being debated [3-5]. Bearing in mind that, as a result of treatment and the disease itself, drastic changes occur in the different components of physical loss, body composition, and quality of life, it is necessary to find strategies capable of minimizing the negative effects and, consequently, improving the long-term quality of life of these individuals [4-6].
Physical activity has been suggested as a tool to reduce the negative effects, being considered a useful, safe, and effective non-pharmacological therapy with increasing influence [6]. In this context, both aerobic and resistance training are recommended for this population, taking into account that they encompass different mechanisms, and combining them during exercise sessions brings even more benefits than practicing them separately [7]. Several systematic reviews and meta-analyses have found positive effects of exercise on the quality of life, psychological well-being, and physical outcomes of cancer survivors [8-15]. However, these systematic reviews did not focus on the effects of concurrent exercise (also known as combined exercise). Therefore, this update of scientific evidence aimed to synthesize and analyze the scientific evidence on the effectiveness of concurrent training on physiological, psychological, and social variables, as well as on adverse effects related to concurrent training in cancer or cancer survivors. Our aim was to contribute to a theoretical debate and offer suggestions for future research in this area of work.
Two research questions were considered for the creation of this update of scientific evidence, specifically i) What are the physiological effects of concurrent training in people with cancer?; ii) What are the psychological effects of concurrent training in people with cancer?
The ISI Web of Science, Scopus, and PubMed databases were used to search for scientific articles between 2000 and 2023 for this update of scientific evidence. This article included studies on the effect of concurrent training in the same training session on people with cancer. In this search, the articles were identified using the following keywords, individually and/or in combination (Table 1).
Table 1: Keywords and search terms used
|
Set |
Search terms |
|
1 |
((("Cancer Survivors") OR "Breast Cancer Lymphedema") OR "Breast Neoplasms") OR "Medical Oncology" |
|
2 |
(("Exercise") OR "Resistance Training") OR "Exercise Therapy" OR "concurrent training" OR "combined training" |
|
1+2 |
((("Exercise") OR "Resistance Training") OR "Exercise Therapy" OR "concurrent training" OR "combined training") AND (((("Cancer Survivors") OR "Breast Cancer Lymphedema") OR "Breast Neoplasms") OR "Medical Oncology") |
|
Systematic Review and meta-analyze |
((("Exercise") OR "Resistance Training") OR "Exercise Therapy" OR "concurrent training" OR "combined training") AND (((("Cancer Survivors") OR "Breast Cancer Lymphedema") OR "Breast Neoplasms") OR "Medical Oncology") |
Studies were included in this update of scientific evidence if they met the following selection criteria: i) articles written in English; ii) published in peer-reviewed journals; iii) research questions on the effect of concurrent training on cancer. The exclusion criteria were: i) Non-systematic reviews with meta-analysis were not included; ii) studies that do not report means, standard deviations, and significance values; iii) studies carried out online; iv) articles in which the effect of concurrent training was not in the same training session.
Data from the included studies were analyzed by two independent reviewers and extracted into Microsoft ExcelTM. Any discrepancies were resolved through discussion with a third author. The methodological quality of all the included studies was assessed using the quality assessment tool developed by Hawker and colleagues [16]. The tool is composed of nine components to assess the methodology of the studies, examining the introduction and aims; methodology; sampling; data analysis; ethics and bias; results; transferability and generalisability of the findings; and the implications and usefulness of the findings for policy and practice. Each component is assessed according to a four-point Likert scale (good, fair, poor, and very poor), and numerical values are assigned to each scale (overall score range: 9-36), with an overall score of 36 indicating studies with rigorous methodologies (Table 2). This update of the scientific evidence was carried out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Table 2: Quality Assessment of included study [17]
|
Item |
Quality assessment |
|
Abstract |
Good |
|
Introduction and aims |
Good |
|
Methods and data |
Good |
|
Sampling |
Good |
|
Data analysis |
Good |
|
Ethics and bias |
Good |
|
Results |
Good |
|
Transferability and generalizability |
Good |
|
Implications and usefulness |
Good |
|
Overall Score † |
36 |
|
† Very Poor =1; Poor =2; Fair = 3; Good =4 points. Score range = 9-36 (minimum- maximum).
|
|
Study Characteristics
The preliminary search identified 2181 studies from 3 different databases. A total of 1660 articles were identified after removing duplicates. After reading the titles and abstracts, the vast majority of the articles were not relevant. We selected 201 articles whose titles and abstracts satisfied our inclusion criteria. Those who were irrelevant or did not meet the inclusion criteria were eliminated. A total of 1 study satisfied the pre-defined inclusion criteria on the effect of concurrent training on cancer pathology. This article used aerobic exercise training (AET) plus resistance exercise training (RET). Tables 3 and 4 give a detailed account of the studies included, showing the study population, type of frequency, type, duration, intensity, and outcomes (Figure 1).
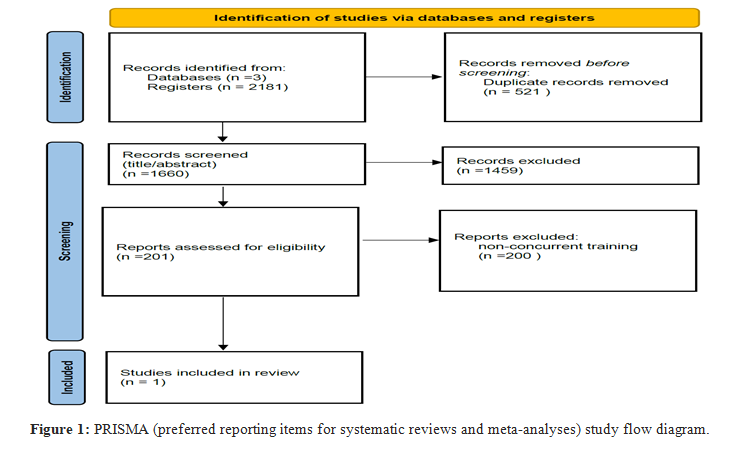
The update of the scientific evidence included 8 studies. The study sample size includes 470 participants, 252 controls, and 218 trials (AET + RET). The population included in this article is made up of people with breast cancer. Of the articles included, 6 articles are in phases I to III, 1 article in phases I and II, and 1 article in phase V. The studies were conducted in the USA, Spain, China, New Zealand, Sweden, Iran and Australia.
The interventions for concurrent training in people with cancer range from 2 weeks to 23 months and the follow-up ranges from 6 to 12 months. The frequency of intervention in this population and this training method varies from 2 to 4 sessions/week. According to the article selected for an update of the scientific evidence, aerobic exercise training uses the Cycle ergometer and brisk walk, lasting between 10 and 90 minutes and with intensities between 50-80 HRmax. As for resistance exercise training, neither the type of exercise nor intensity is mentioned. Resistance exercise training is performed in 1 to 3 sets of 8 to 20 repetitions, lasting between 15 and 60 minutes.
The outcomes analyzed in the update of the scientific evidence were peak oxygen uptake, peak heart rate, peak respiratory exchange ratio, maximal oxygen consumption, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol, triglycerides, and body mass index. According to the results presented in the only systematic review included, interventions based on concurrent training have positive effects on increasing VO2peak, VO2max, and decreasing TG. However, there were no significant differences in HRpeak, RERpeak, SBP, DBP, HDL-C, and BMI using concurrent training. The description of the reviews included and interventions are presented in Table 3 and Table 4.
Table 3: Description of systematic reviews included [17].
|
Years |
Sample size, N |
Cancer type |
Participant characteristic |
Intervention time |
Location |
|
2006 - 2021 |
8 Studies |
Breast Cancer 6 articles (stages I to III) 1 article (stages I and II) 1 article (stages Iv) |
470 samples 252 controls 218 experiments (AET?+?RET)
46.7?±?10.0 years 65.0?±?6.9 years |
8 to 16 weeks 6–12 months of follow-up |
USA, Spain, China, New Zealand, Sweden, Iran, Australia |
AET = aerobic exercise training, RET= resistance exercise training
Table 4: Interventions included in the review [17].
|
Frequency |
Aerobic exercise training (AET) |
Resistance exercise training (RET) |
Outcomes |
||||||
|
Type |
Duration |
Intensity |
Type |
Duration |
Set |
Repetitions |
Intensity |
||
|
2-4 sessions/week |
Cycle ergometer and brisk walk |
10- 90 min |
50-80 HRmax |
NS |
15-60 min |
1-3 |
8-20 |
NS |
VO2peak, HRpeak, RERpeak VO2max SBP, DBP, HDL-C, TG, BMI |
|
BMI = body mass index, DBP = diastolic blood pressure, HDL-C = high-density lipoprotein cholesterol, HRpeak = peak heart rate, NS = not specified, RERpeak = peak respiratory exchange ratio, NS = not specified, SBP = systolic blood pressure, TG = triglycerides, VO2max = maximal oxygen consumption, VO2peak = peak oxygen uptake. |
|||||||||
This update of the scientific evidence analyzed the effect of training on cancer survivors, focusing on the methodology used, training methods, training periods, and outcome measures. Despite the exponential development of research into exercise and cancer in recent years, research into the effect of concurrent training is still scarce. This update of the scientific evidence included only 1 article. This study involved breast cancer patients diagnosed with stages I to V. The outcomes analyzed in the systematic review were VO2peak, HRpeak, RERpeak, VO2max, SBP, DBP, HDL-C, TG, and BMI. The main finding regarding the effects of concurrent training on cancer was that it had positive effects on increasing VO2peak, VO2max, and decreasing TG. However, there were no significant differences in HRpeak, RERpeak, SBP, DBP, HDL-C, and BMI with the use of concurrent training.
Research and interest in oncological exercise have been increasing, reinforcing the importance of aerobic and resistance training [8]. Due to the complexity, heterogeneity, and symptoms of the different cancers, we cannot conclude that aerobic training is more beneficial than resistance training, or vice versa [18]. Both aerobic and resistance exercises are effective as a non-pharmacological treatment for cancer [18]. Aerobic and resistance exercise seems to improve the physiological and psychological components and increase the quality of life of cancer patients [16,19-21]. These two training methods can improve cardiopulmonary function, muscle strength, body composition, bone density, quality of life, changes in some blood markers, less feeling of fatigue and reduce the toxic effects of treatment [16,19,22-28]. Due to the many benefits of both aerobic and resistance training, the concurrent training methodology is increasingly being used as an intervention for people with cancer. Concurrent training thus aims to combine the two types of aerobic and resistance training in the same training session, enhancing the non-pharmacological effect of both workouts. However, there is a lack of general research into the effects of this methodology on improving quality of life, fatigue, cardiorespiratory capacity, strength, body composition, bone density, and changes in blood markers. This methodology still lacks a general definition of frequency, duration, intensity, exercises, and order of methodologies in this type of training.
Based on the results of this article, we recommend future studies, long-term research, and larger sample sizes, to verify the effect of this type of intervention on other types of cancer. More research is needed into the types of exercise, frequency, duration, intensity, and the effect of changing the order of aerobic and resistance exercises. We also suggest checking the effect of the intervention on physiological and psychological factors such as cardiorespiratory capacity, muscle strength, body composition, blood factors, quality of life, and fatigue.
Both aerobic and resistance training present many benefits in cancer treatment and survivorship, therefore, concurrent training is increasingly being used. Nevertheless, there is little general evidence on the intervention of concurrent training among this population, so there is a need for original studies investigating the main characteristics and positive effects of concurrent training. However, interventions based on concurrent training appear to have positive effects on increasing VO2peak, VO2max, and decreasing TG. On the other hand, this type of training did not show differences in HRpeak, RERpeak, SBP, DBP, HDL-C, and BMI.
This work was supported by FCT—Portuguese Foundation for Science and Technology, through the project UIDB/DTP/04045/2020. This work was developed within the scope of the CICS-UBI and CIDESD.
The author declares that there is no conflict of interest.